
Welcome to this series on analytical techniques for research! In this series, we will talk about the essential techniques that you might come across when dealing with materials-based or product-based research. While the synthesis of such products and materials is an important aspect to look at, it is also one thing to check for the identity and the properties of your product. This article will serve as an introduction to what analysis is and why is it important.
What is (Chemical) Analysis?
When we have our results or products, the next thing we need to do is to analyze them. There are various types of analysis that we perform on data and products. To analyze data, we have statistical analysis to deal with that- but what about products? This is where chemical analysis comes in- just to be clear, chemical analysis is just one of the ways we analyze products. Other fields may have their own methods of analysis, but chemical analysis is widely usedfor natural products research, synthetic research, and a lot of other fields.

How to Analyze Samples?
In chemical analysis, we test the physical and chemical properties of various compounds- in this case, we call the products that we analyze as our analyze. This is done with the aid of various techniques, and in most cases, machines too. Usually, chemical analysis can be split either according to qualitative and quantitative analysis or classical and instrumental analysis.
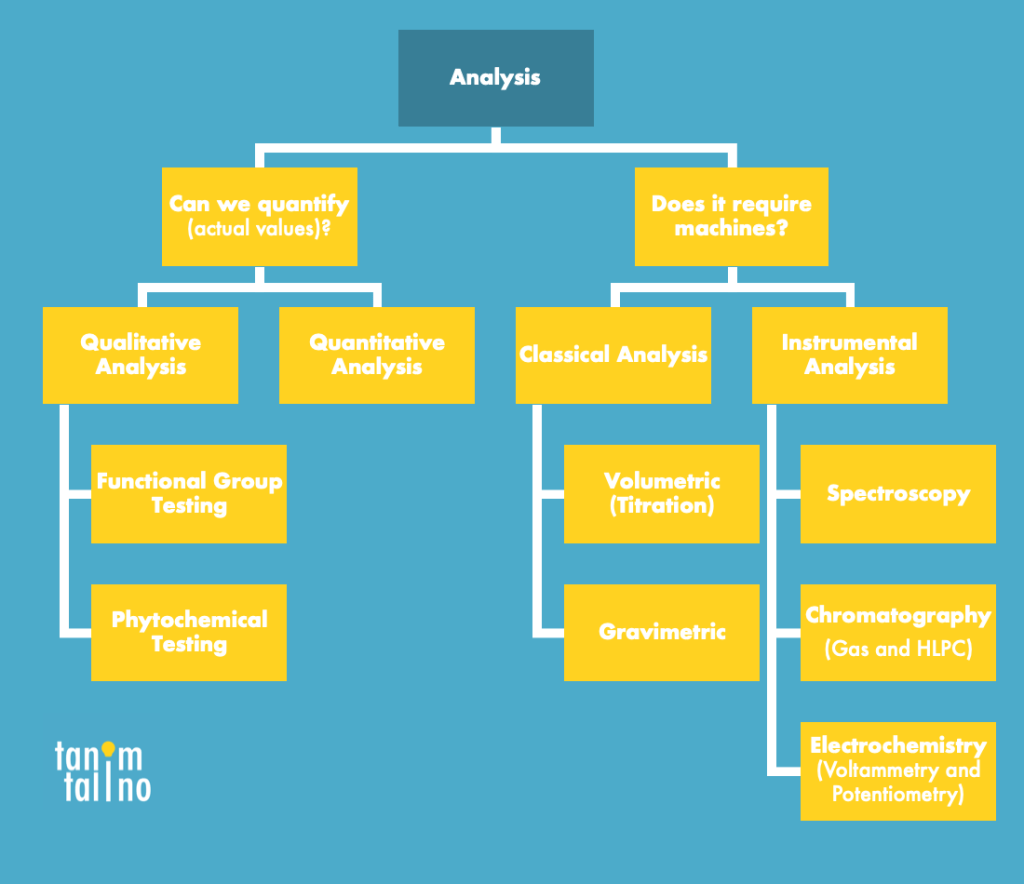
In qualitative analysis, we perform tests that give out non-quantitative or non-numerical information about our sample. Because of this, we rely on property changes. One prominent example of this is the Iodine Test. In this test, we add liquid iodine to detect for the presence of starch in our sample- if the sample turns dark blue, then we can say that starch is present.
In qualitative analysis on the other hand, we deal with measurements and numerical data. For example, we try to calculate and find the concentration of a certain chemical species. A simple example of this is titration, where we rely on the neutralization of a sample of unknown concentration with that of a titrant with a known concentration. For example, to know the concentration of vinegar, we can titrate it with sodium hydroxide of known concentration. We will deal with this more when we get to volumetric analysis.
Between classical and instrumental analysis, we usually differentiate this with the use of sophisticated instrumentation. Classical analysis often deals with reactions rather than machines to analyze the sample. There are two types of classical analysis: gravimetric and volumetric analysis. In the former, we try to use mass in order to analyze the sample- this may seem simple, but we have to take note that analyses are often contaminated or have certain byproducts and thus numerous steps have to be taken to isolate the analyte. In the latter on the other hand we use solutions, where weight is not appropriate to be used in this case. Rather, we use the volume of these solutions and their calculations. One example of volumetric analysis is titrations which was discussed earlier.
Nowadays, there are also novel analytical methods that combine chemistry with other fields. For example, we now have immunoassays and antimicrobial assays, where we check for the product’s activity against antibodies/antigens and microbes, respectively.
In the coming modules, we will discuss some of the most prominent analytical techniques that are needed for chemistry and materials science research, so stay tuned as we update Learn with further content!

- We use chemical analysis to know the product’s identity and properties.
- Chemical analysis involves both qualitative and quantitative methods, with qualitative analysis focusing on property changes and quantitative analysis dealing with numerical data.
- Classical analysis relies on reactions and often involves gravimetric or volumetric techniques, while instrumental analysis utilizes sophisticated instrumentation.